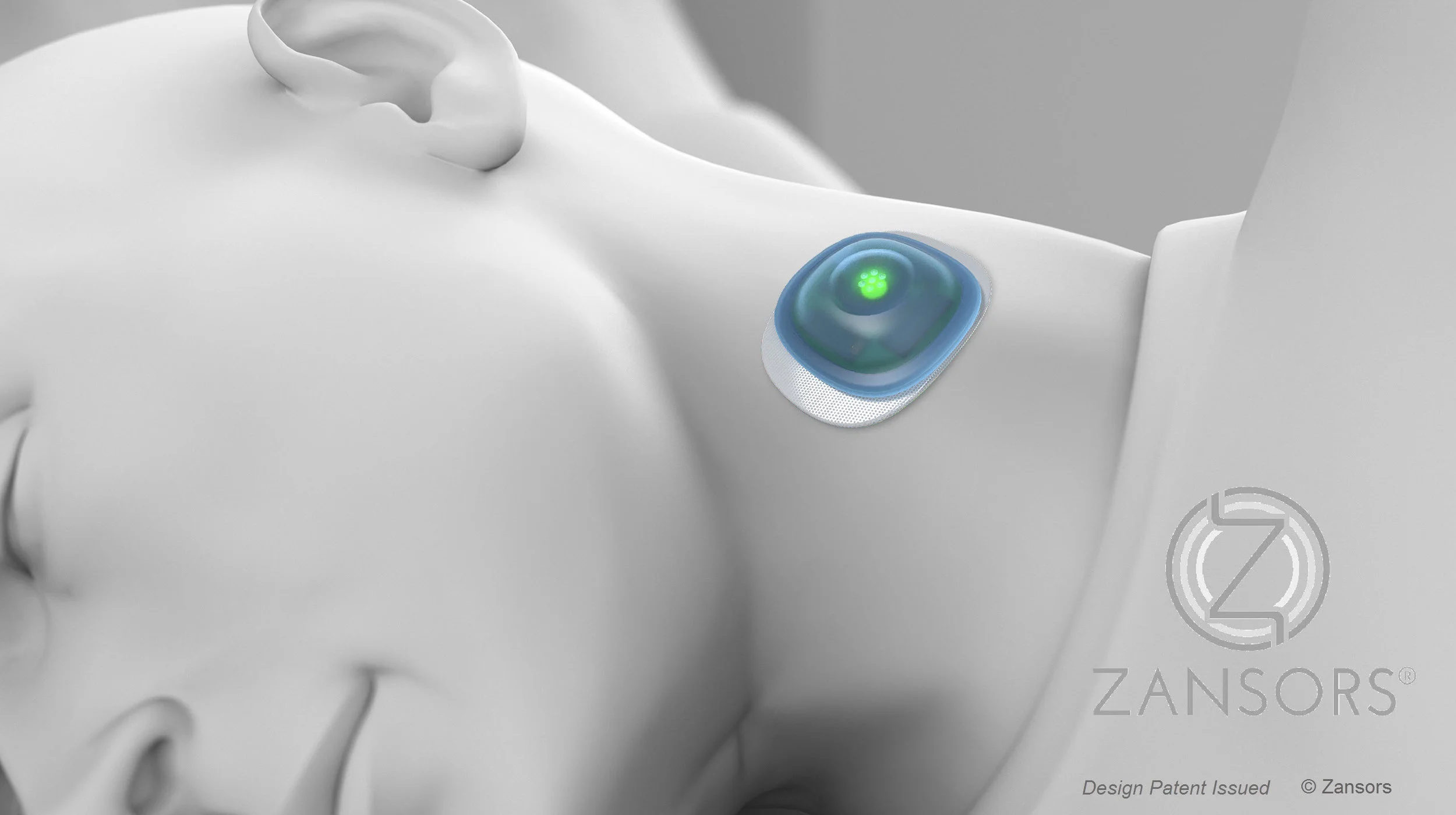
Zansors Wins NIH Grant to Advance Wearable At-Home Affordable Sleep Apnea Detection
This additional, larger, Phase II grant from National Institutes of Health will continue support of research and commercialization of Zansors’ “first to market” wearable consumer sleep-apnea product
Arlington, VA. (January 25, 2017) -- Zansors, a healthcare innovation company focused on wearable bio-sensors and health apps, was awarded $1.5 million by the NIH's National Institute on Minority Health and Health Disparities (NIMHD) for Phase II of their SBIR/STTR program. This is a continuation of a previously funded Phase I grant and is Zansors’ sixth grant win from NIH.
The funds will be used to further develop research and then seek FDA approval for a wearable sleep product to screen for sleep apnea. This would be a wireless, wearable, over the counter sleep apnea product. More than 18 million adult Americans have sleep apnea, according to the National Sleep Foundation. Up to 80 percent of these cases may be undiagnosed. Untreated sleep apnea can lead to conditions from hypertension and heart disease to tiredness and depression.
“With this funding, our aim is to significantly increase the screening of at-risk, under-served individuals by making diagnosis easy and affordable for all.” said Todd Arnedt, PhD, Director of the Insomnia and Behavioral Sleep Medicine Program, University of Michigan.
Dr. Arnedt will serve as Principal Investigator for the grant, and University of Michigan will have a significant role in this research. Abhijit Dasgupta, PhD, Co-founder and Chief Data Science Officer of Zansors, will serve as Co-Investigator.
“We are using our technology to combine wearable sensors, algorithms and bioengineering into a first of its kind product,” said Dr. Dasgupta. “The process to create this sleep apnea screening device is complex, but the idea is simple: Zansors making affordable, easy-to-use diagnostic tools so people can be proactive about their health. You can’t improve yourself without first identifying the issues.”
Phase I research on this sleep apnea device assessed feasibility, and the device was tested on 50 patients at University of Michigan. This phase also demonstrated that the device performs well compared to gold-standard diagnostics for sleep apnea. These early positive results have helped Zansors gain support from leading healthcare institutions, including Kaiser Permanente and Inova Health System.
Phase II, which is currently in progress, will focus on validation, beginning with improving the design and bio-engineering of the device and developing more efficient and accurate algorithms.
“This technology not only holds promise for helping Americans detect sleep apnea, it also has great potential to decrease costs in healthcare,” said Mark Fendrick, MD, Professor of Internal Medicine and Health Management and Policy, and Director of the Center for Value-Based Insurance Design at the University of Michigan. “The research that NIMHD is funding is exactly what is needed to introduce a more effective way of delivering care.”
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Research reported in this press release was supported by National Institute on Minority Health and Health Disparities of the National Institutes of Health under award number: 2R42MD008845-02.
About Zansors
Zansors is a Washington, DC metro-based healthcare innovation company focused on wearable bio-sensors and health apps. Driven by our credo of “know yourself”, Zansors combines evidence-based apps, bio-engineering and data analytics, allowing consumers to take charge of their health. Our proprietary biotechnology platform includes micro-sensors and micro-fluidics (also known as “lab-on-a-chip”) backed by a strong portfolio of patents filed. Zansors has previously received 5 funding awards by NIH and has formal partnerships with the University of Michigan and George Washington University.